Abstract
Background: CT-P10 is the first biosimilar to innovator rituximab (RTX) approved for all indications by the European Medicines Agency. The pharmacokinetics (PK) equivalence and non-inferior efficacy of CT-P10 compared with RTX in patients with newly diagnosed advanced follicular lymphoma (FL) was previously demonstrated when used with combination chemotherapy of cyclophosphamide, vincristine and prednisone (CVP) (Kim WS et al,. 2017).
Objectives: This is an ongoing, phase 3, randomized, double blind, active controlled study to demonstrate similarity of efficacy and safety between CT-P10 and RTX as monotherapy in patients with newly diagnosed FL with low tumor burden (NCT02260804). The results of efficacy as determined by overall response rate (ORR), PK, pharmacodynamic, safety and immunogenicity up to 7 months (prior to Maintenance Cycle 3) are presented here.
Methods: Patients with FL with stage II-IV, Grade 1-3a and low tumor burden as assessed by GELF criteria were randomized in a 1:1 ratio to receive either CT-P10 or RTX (375mg/m2 i.v.) monotherapy weekly for 4 weeks as induction therapy, followed by maintenance therapy every 2 months for 2 years. Patients who had complete response (CR), unconfirmed CR, partial response or stable disease after the induction therapy were eligible for maintenance therapy. The primary endpoint was ORR over 7 months according to the IWG criteria and was assessed by the independent review committee as central review and by the site investigator as local review.
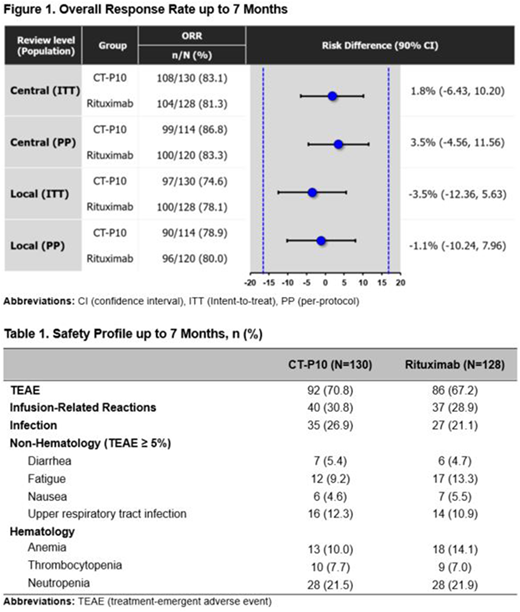
Results: In total, 258 patients were randomized to either the CT-P10 (n=130) or RTX (n=128) group with well-balanced baseline characteristics. Overall, 231 patients (n=119/130 [92%] and n=112/128 [88%] in the CT-P10 and rituximab groups, respectively) completed up to 7 months. Therapeutic similarity between CT-P10 and RTX was established by meeting the predefined margin of ±17% for the primary efficacy endpoint of ORR in the intent-to-treat (ITT) and per-protocol (PP) population (Figure 1). An ORR of 83.1% and 81.3% for CT-P10 and RTX, respectively, was observed over 7 months. The ORR difference was 1.8% and 90% confidence interval (CI) of the treatment difference estimate was entirely within the equivalence margin; 90% CI (-6.43, 10.20).
Similar PK profiles were shown in the CT-P10 and RTX. Maximum and trough concentration of CT-P10 or RTX at each cycle were similar between the two groups. Median number of B-cells decreased to the lower limit of quantification (<20cells/μL) after the 1st infusion and remained as depleted over 7 months in both groups.
CT-P10 was well tolerated and the safety profile of CT-P10 over 7 months was consistent with that of RTX, with no new, unexpected safety findings. Treatment-emergent adverse events (TEAEs) were reported in 71% and 67% patients in the CT-P10 and RTX, respectively. Adverse events of special interest included infections and infusion-related reactions occurred in a similar proportion of patients in each treatment group (Table 1). Neither progressive multifocal leukoencephalopathy nor Hepatitis B virus reactivation was reported in either group. The proportion of patients with positive anti-drug antibody were similar between 2 groups (0.8% vs. 2.3%) over 7 months. Among them, neutralizing antibody were detected in one patient in the CT-P10 group.
Conclusions: This study demonstrates therapeutic equivalence of CT-P10 to RTX in previously untreated LTBFL. CT-P10 was well-tolerated and the safety profile including immunogenicity of CT P10 was comparable to that of RTX over 7 months.
Ogura:SymBio: Research Funding; Mundi Pharma: Consultancy; Celltrion: Consultancy, Research Funding; Takeda: Honoraria; Cellgene: Honoraria; MeijiSeika Pharma: Consultancy. Sancho:SERVIER: Honoraria; SANOFI: Honoraria; CELGENE: Honoraria; KERN FHARMA: Honoraria, Speakers Bureau; GILEAD: Honoraria, Research Funding; JANSSEN: Honoraria, Speakers Bureau; ROCHE: Honoraria, Speakers Bureau; MUNDIPHARMA: Honoraria. Lennard:Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding. Jurczak:European Medicines Agency: Consultancy; Astra Zeneca/Acerta: Consultancy, Research Funding; Sandoz-Novartis: Consultancy; Janssen: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Afimed: Research Funding; Bayer: Research Funding; Beigene: Research Funding; Celgene: Research Funding; Epizyme: Research Funding; Nordic Nanovector: Research Funding; Merck: Research Funding; Morphosys: Research Funding; Pharmacyclics: Research Funding; Servier: Research Funding; Roche: Research Funding; TG Therapeutics: Research Funding. Coiffier:CELGENE: Consultancy, Membership on an entity's Board of Directors or advisory committees; MUNDIPHARMA: Membership on an entity's Board of Directors or advisory committees; CELLTRION: Membership on an entity's Board of Directors or advisory committees; MORPHOSYS: Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Membership on an entity's Board of Directors or advisory committees. Buske:Roche: Honoraria, Research Funding; Bayer: Research Funding; Janssen: Honoraria, Research Funding. Lee:Celltrion, Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal